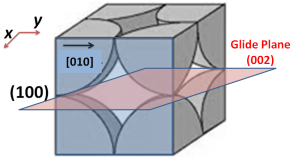
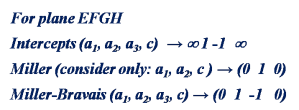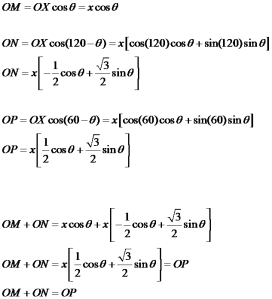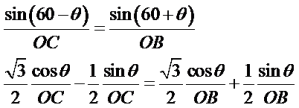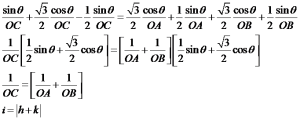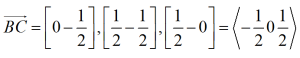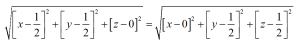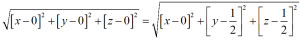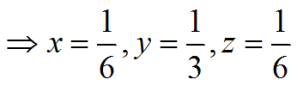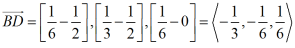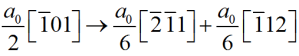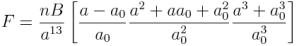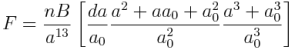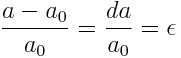In hexagonal lattice (and crystals) directions and planes are designated by the 4-index notations (hkil) called as Miller-Bravais (M-B) notation.
In this post, the importance of M-B notations and derivation for i=-(h+k) is discussed.
Before touching to the aforementioned problem, let’s understand the hexagonal system itself.
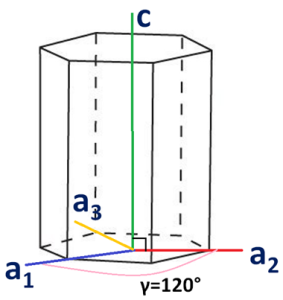
As we all know Miller indices of a hexagonal system are
a=b≠c and α=β=90°, γ=120°
Please note that in the M-B notation (i.e. hkil)
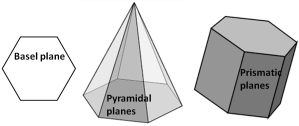
- the first three indices (i.e. h, k and i) are symmetrically related (i.e. to a1, a2 and a3 respectively) to the basal plane
- the third index (i) is a redundant one (i.e. can be derived from the first two and we will see the derivation of it in later part of this post)
- the fourth index (l) represents the ‘c’ axis (perpendicular to the basal plane)
The importance of the M-B notations is to clearly distinguish the family of planes and directions.
The M-B notations are introduced to make sure those members of a family of directions or planes are unambiguously identified.
Just check the following examples.
Consider the prismatic planes ABCD and EFGH. It is obvious and apparent from the following figure that these two planes belong to the same family (as they are parallel to common direction “c”).
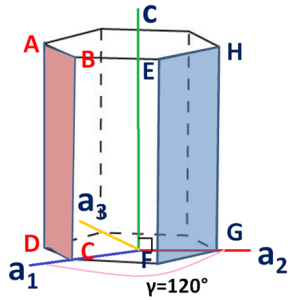
The following figures show the projections of these two planes on basal plane (with lattice parameters “a” and “c” i.e. |a1|=|a2|=|a3|=a and |c|=c).
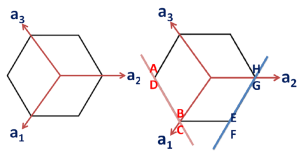
Check out the Miller indices for these two planes.

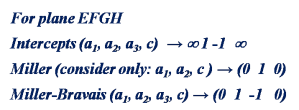
From the above example, it is clear that Miller indices indicate that these two planes are of a different family (even though they belong to the same family). But Miller-Bravais notations confirm that they are from the same family.
Now we will see the relation between the redundant index i with that of h and k, i.e. now we will derive relation i=-(h+k)
As the direction c is parallel to the direction z. So we will not worry, as index notation l in Bravais notations will be equal to that of Miller-Bravais index notation.
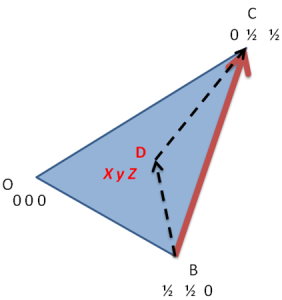
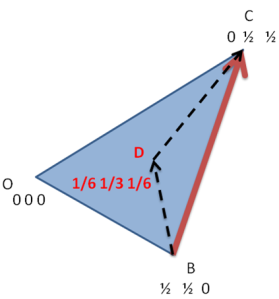
Now consider the basal plane and point X with indices hkil as shown in below figure. Here we will first derive a relation for a direction and same analogy can be extended to planes.
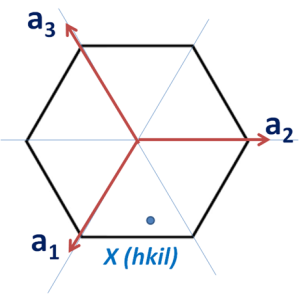
To find out the coordinates of point X i.e. the values of h, K and i in terms of a1, a2 and a3. Thus we have to draw perpendiculars to these three axes which are shown in the below figure.
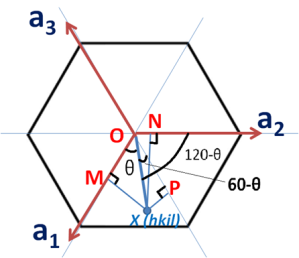
Let the distance OX=x units and then from the above figure
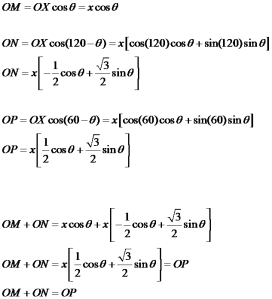
As from the above the direction of OP is opposite to that of a3, thus as per the conventions
OM+ON=-OP
From figure we can say that, OM=h, ON=k and OP=-i
i.e. i=-(h+k)
This we have proved for the direction, with the same analogy, we can proceed for planes. In the case of planes, we have to consider intercept on the respective axis.
As mentioned earlier we are not bothered about the index in z direction as it is going to remain same in the M-B notations.
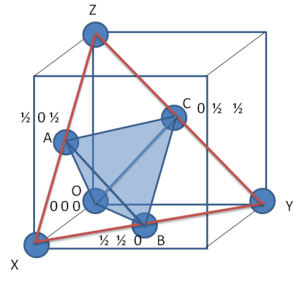
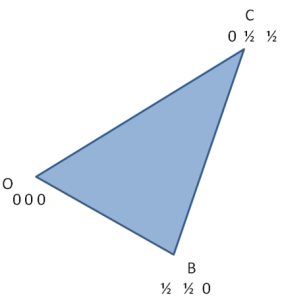
With this understanding, let us consider the plane (marked as a blue line in the following figure) cuts a1 at A, a2 at B and a3 at C.
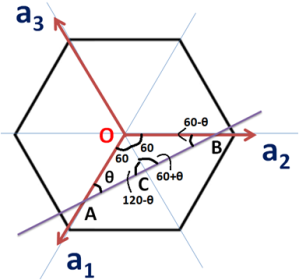
Thus by definition of Miller-indices (/M-B indices)

Now consider ∆OAC, and using law of sines, we can write following relations

Consider above expression as equation (1)
Similarly in ∆OCB,
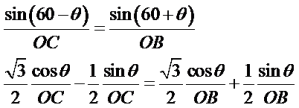
Consider above as equation (2)
Now, add equation (1) and (2)
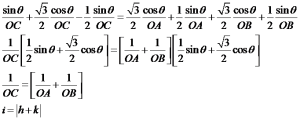
Now as direction of OC is opposite to that of a3
Thus i=-(h+k)
Thus we have proved the relation for both directions and planes. Note that this relation is valid only for perfect hexagon system.